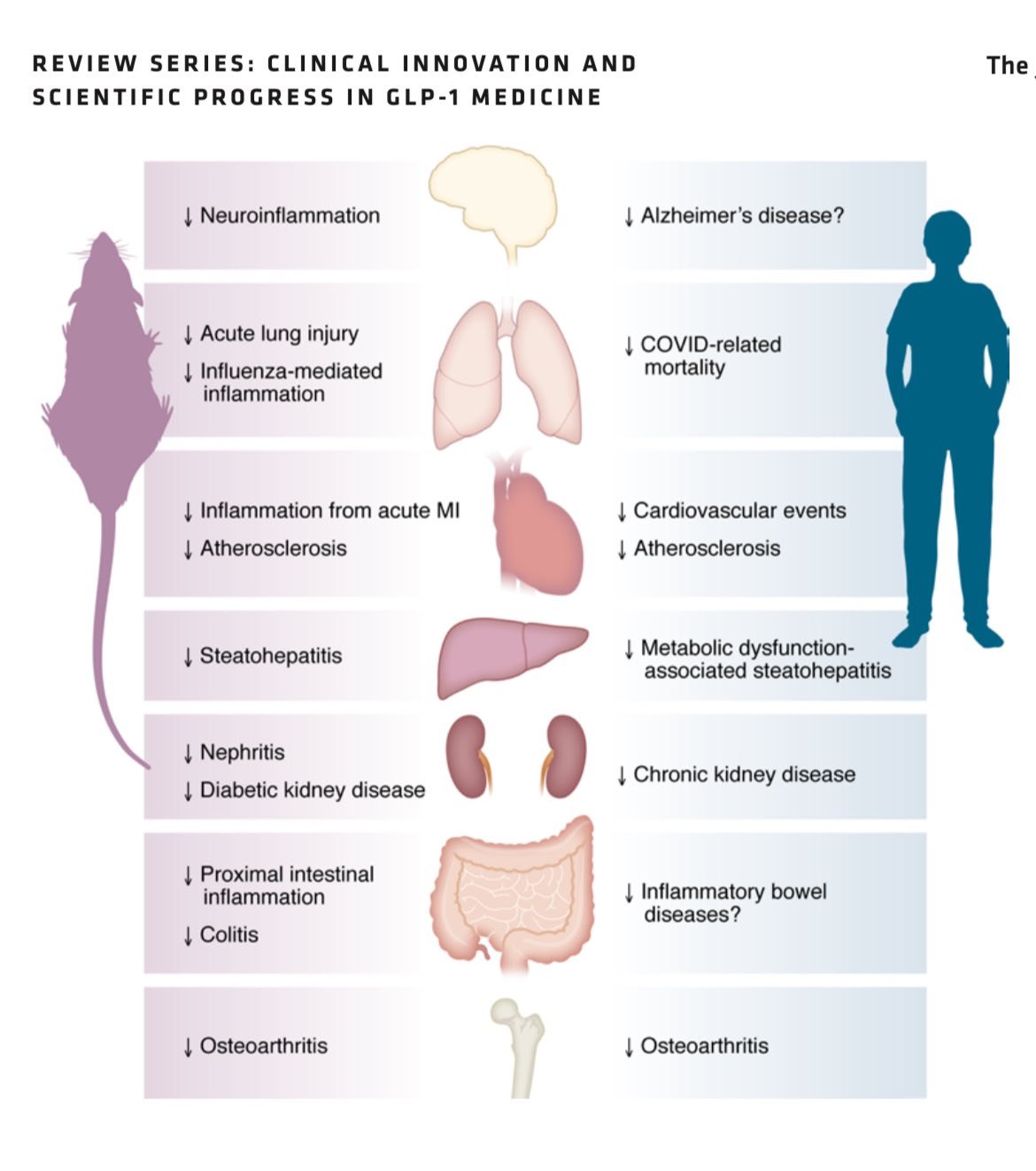

GLP-1 agonists like semaglutide (Ozempic, Wegovy) and tirzepatide (Mounjaro, Zepbound) mimic a gut hormone that improves insulin sensitivity, slows digestion, and suppresses appetite — often leading to dramatic weight loss.
Of course, like any medication, they’re not without trade-offs. The most common side effects are gastrointestinal — nausea, bloating, constipation, or reflux — and most people know that part of the story going in.
But there’s a subtler one emerging that I’ve been seeing in my own practice.As these drugs reshape metabolism, they may also be changing muscle, joint, and connective-tissue health in ways we don’t yet fully understand.
I’m starting to notice a new pattern: post-GLP-1 musculoskeletal pain — especially in hips, knees, and backs — that doesn’t behave like a simple “lighter-load” issue.
No prior pain, low baseline activity — mostly walking, light chores, gardening — then new pain showing up after significant weight loss.
It makes me wonder how much of this is related to changes in lean mass and tissue capacity in the context of lower mechanical load and an improved inflammatory profile.
Maybe these people are metabolically “younger,” but mechanically a little more fragile — and that tension between the two might be where some of the mystery lies.
Of course, like any medication, they’re not without trade-offs. The most common side effects are gastrointestinal — nausea, bloating, constipation, or reflux — and most people know that part of the story going in.
But there’s a subtler one emerging that I’ve been seeing in my own practice.As these drugs reshape metabolism, they may also be changing muscle, joint, and connective-tissue health in ways we don’t yet fully understand.
I’m starting to notice a new pattern: post-GLP-1 musculoskeletal pain — especially in hips, knees, and backs — that doesn’t behave like a simple “lighter-load” issue.
No prior pain, low baseline activity — mostly walking, light chores, gardening — then new pain showing up after significant weight loss.
It makes me wonder how much of this is related to changes in lean mass and tissue capacity in the context of lower mechanical load and an improved inflammatory profile.
Maybe these people are metabolically “younger,” but mechanically a little more fragile — and that tension between the two might be where some of the mystery lies.
STEP 1 trial (Wilding et al., NEJM 2021) — the one that sparked much of the global excitement around GLP-1s:
“The mean change in body weight from baseline to week 68 was −14.9 % in the semaglutide group as compared with −2.4 % with placebo… Participants who received semaglutide had a greater improvement with respect to cardiometabolic risk factors and a greater increase in participant-reported physical functioning from baseline than those who received placebo.”
For the first time, a medication could achieve double-digit, sustained weight loss alongside meaningful improvements in health and daily function.
“The mean change in body weight from baseline to week 68 was −14.9 % in the semaglutide group as compared with −2.4 % with placebo… Participants who received semaglutide had a greater improvement with respect to cardiometabolic risk factors and a greater increase in participant-reported physical functioning from baseline than those who received placebo.”
For the first time, a medication could achieve double-digit, sustained weight loss alongside meaningful improvements in health and daily function.
🧪 About the research
The STEP 1 trial — published in the New England Journal of Medicine — was a randomized, double-blind, placebo-controlled study that followed more than 1,900 adults with overweight or obesity (but without diabetes) for 68 weeks.
Participants were randomly assigned to receive either a semaglutide 2.4 mg once weekly or placebo.
Researchers tracked changes in:
It’s one of the most rigorous GLP-1 trials to date — and it’s also where the story starts to get more interesting.
The STEP 1 trial — published in the New England Journal of Medicine — was a randomized, double-blind, placebo-controlled study that followed more than 1,900 adults with overweight or obesity (but without diabetes) for 68 weeks.
Participants were randomly assigned to receive either a semaglutide 2.4 mg once weekly or placebo.
Researchers tracked changes in:
- Body weight
- Metabolic health markers (A1C, insulin, lipids, CRP)
- And in a smaller DXA subgroup (~140 people) — detailed shifts in body composition (fat vs. lean mass).
It’s one of the most rigorous GLP-1 trials to date — and it’s also where the story starts to get more interesting.




